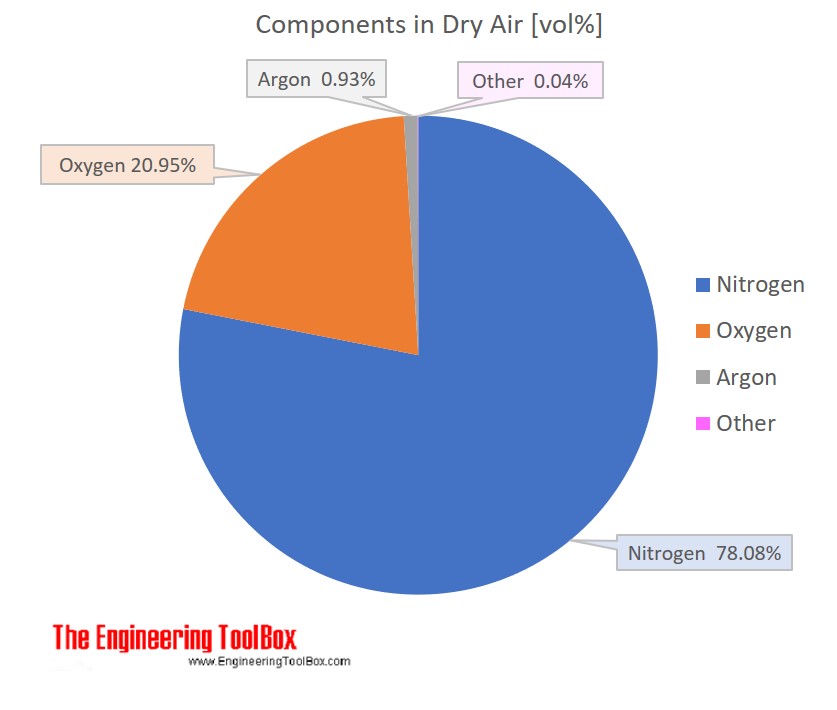
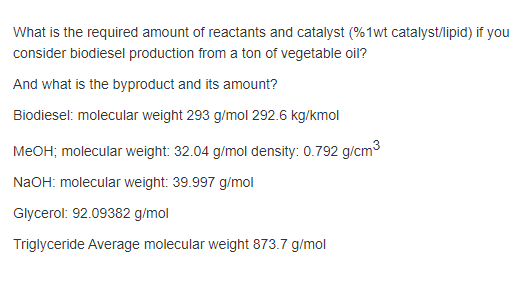
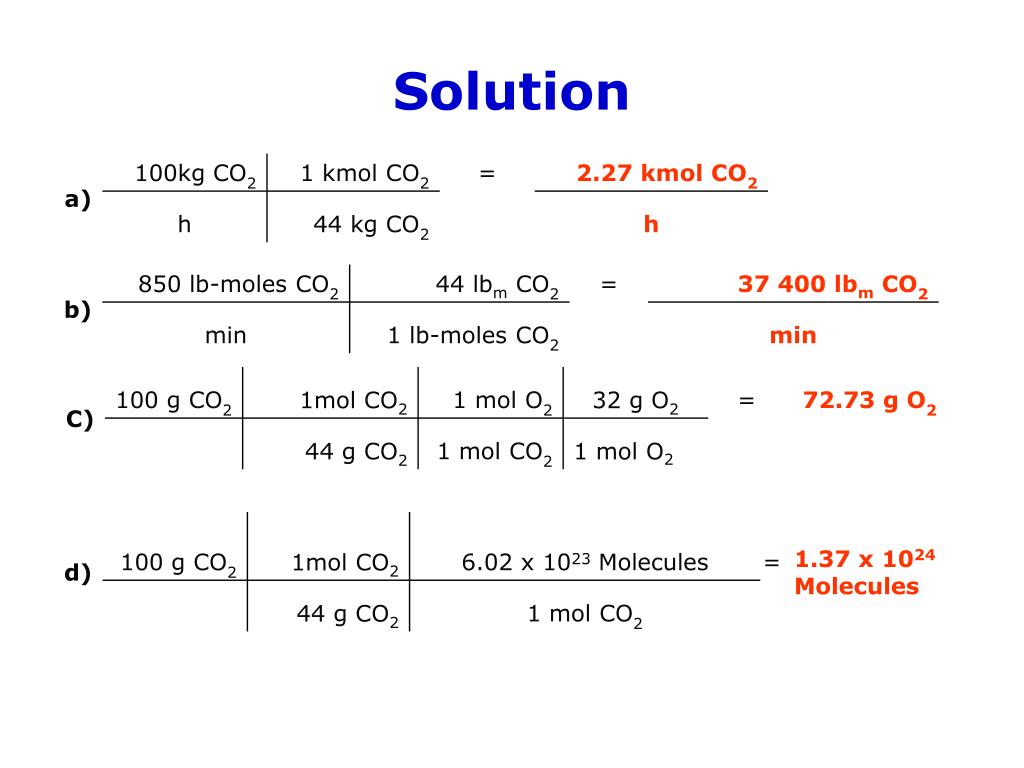
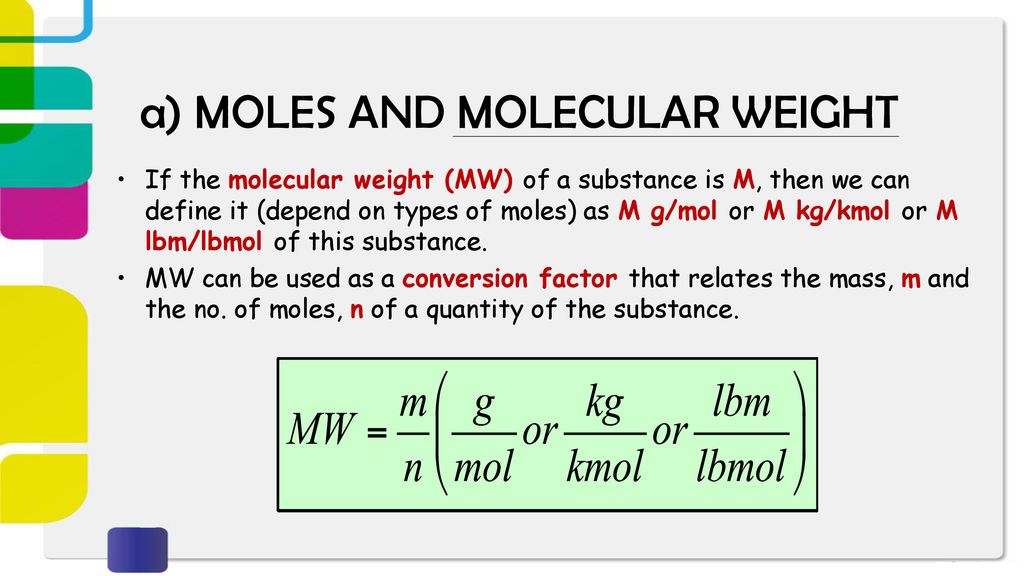
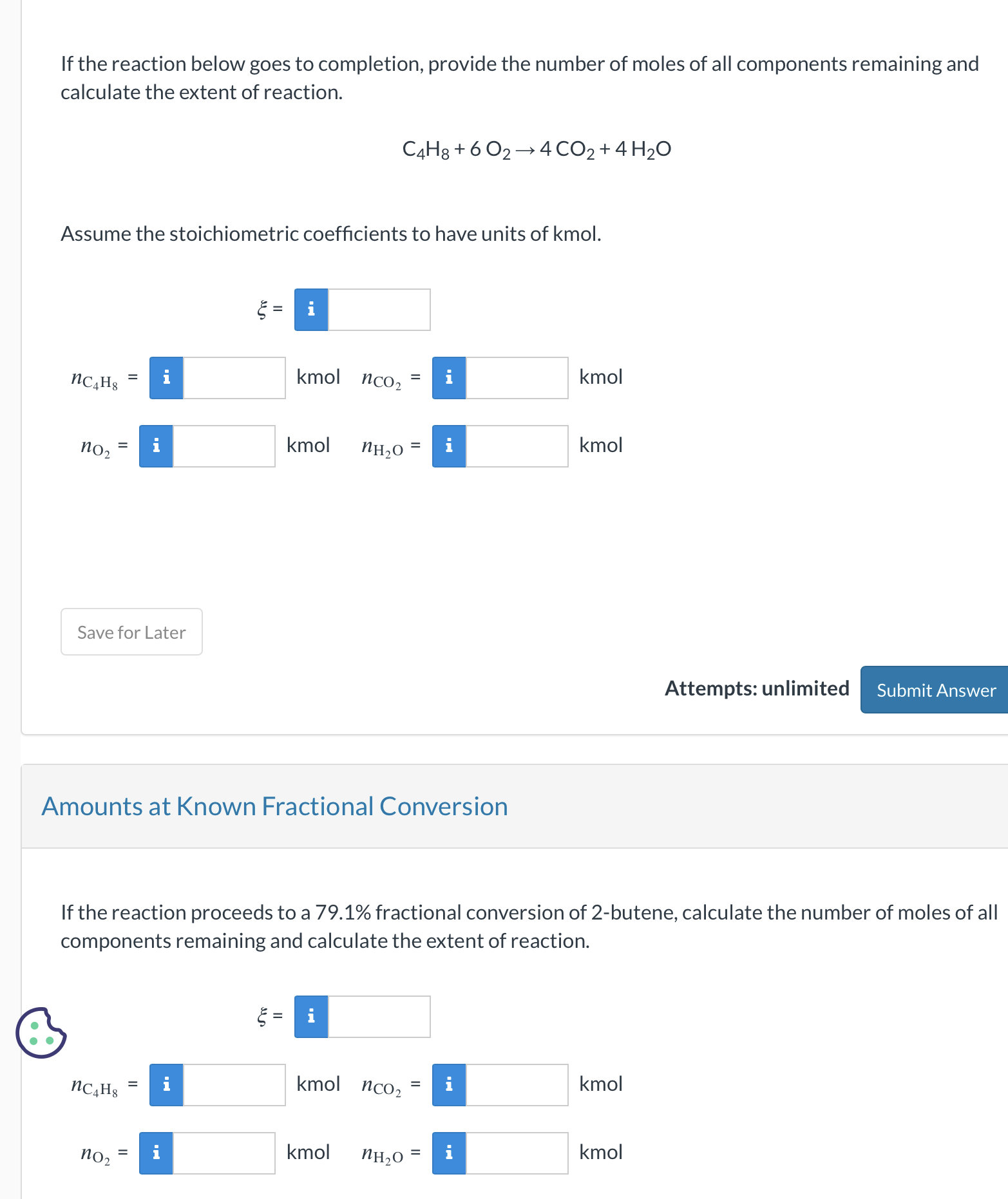
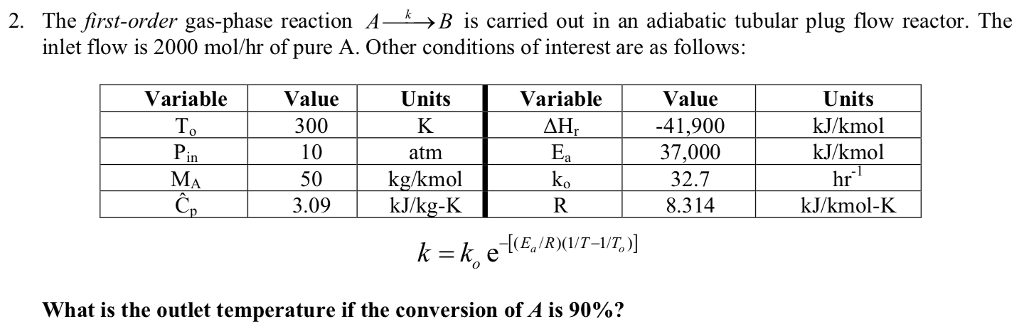
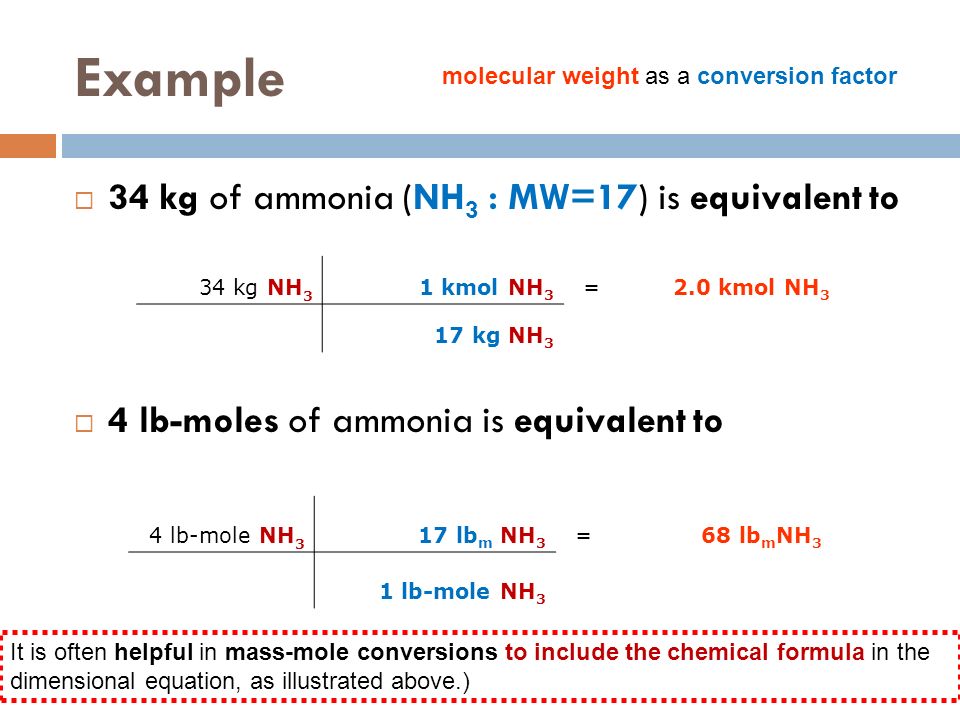
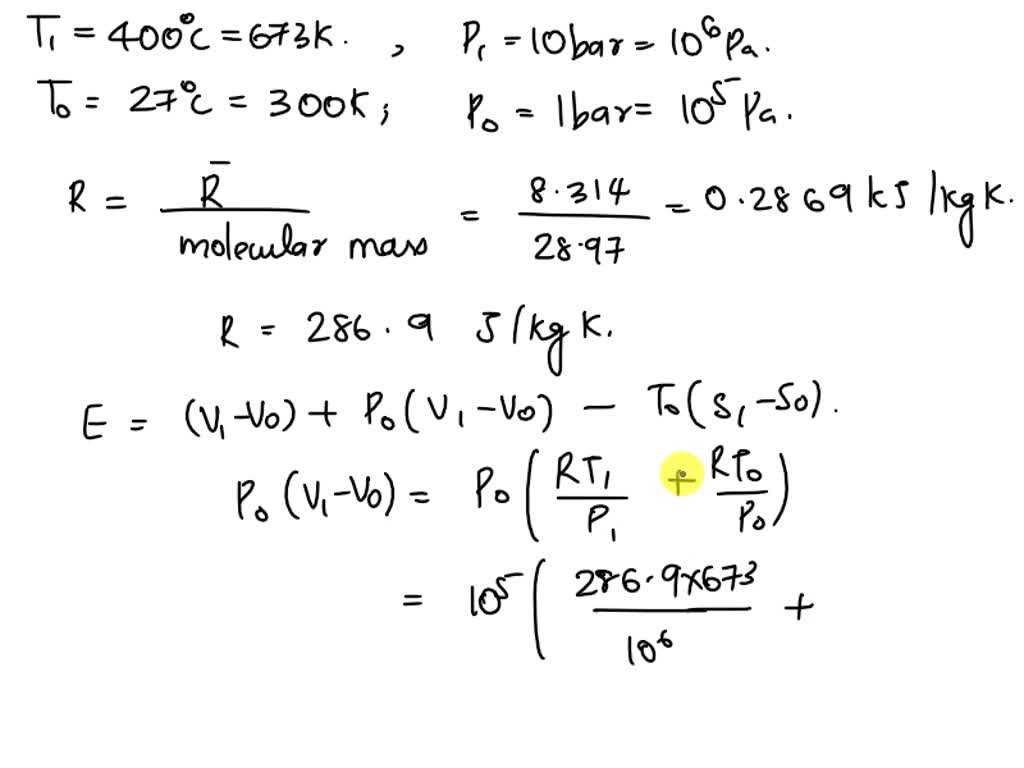SOLVED: Molecular mass of air is 28.97 kg/kmol (Do NOT interpolate, use the closest property values in the ideal gas table). Show detailed work to receive credits. Your score is: For Instructor
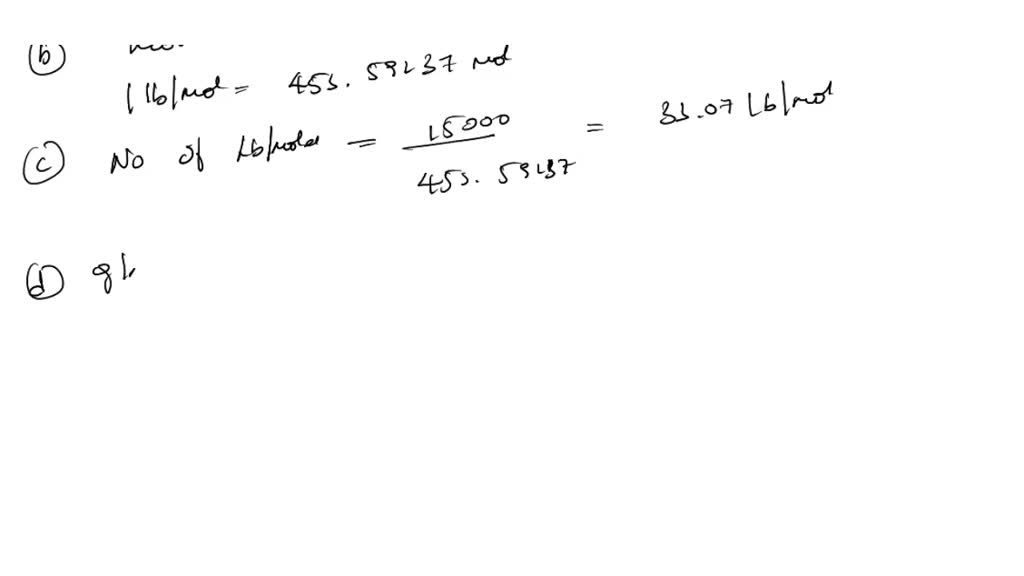
SOLVED: How many of the following are found in 15.0 kmol of benzene (C6H6)? (a) kg CH4; (b) mol C6H6; (c) lb-mole C6H6; (d) mol (g-atom) C; (e) mol H; (f) g
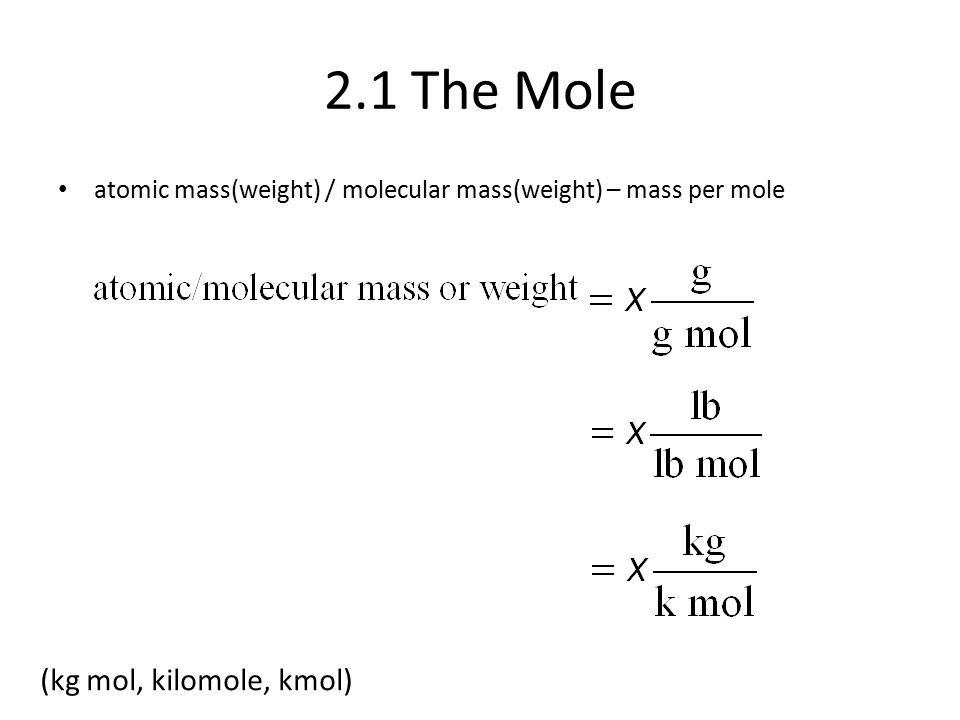
Lecture 2. Moles, Density, Specific Gravity, Fraction, Pseudo-Molecular Weight of Air, Concentration and Flow Rate. - ppt video online download
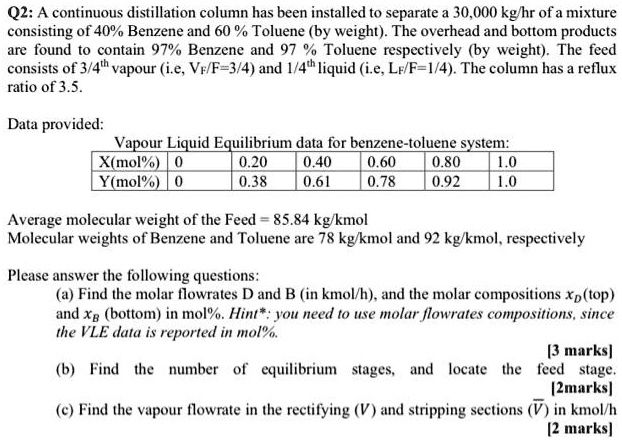
SOLVED: A continuous distillation column has been installed to separate a 30,000 kg/hr of a mixture consisting of 40% Benzene and 60% Toluene (by weight). The overhead and bottom products are found

SOLVED: 7.Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent. This contrasts with the definition of molarity which