
Converting Floating-Point Numbers to Strings in C Without Standard Library Functions | PDF | Integer (Computer Science) | String (Computer Science)

nus. TI I J The amount of heat energy required to convert 1 kg of ice -10°C to water 100°C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat

SOLVED: Calculate the total heat, in joules, needed to convert 15.0 g of liquid ethanol at 25.0 °C to gas at its boiling point of 78.0 °C. Ethanol has a specific heat

Calculate the heat required to convert 3 kg of ice -12°C kept in a calorimeter to steam 100°C atmospheric pressure. Given specific heat capacity ice = 2100 J kg '°C !, specific

A student sets up the following equation to convert a measurement. (The stands for a number the student is - brainly.com

Amazon.co.jp: Overseas Conversion Plug, TESSAN Outlet Conversion Plug, International Travel Power Conversion Plug, Worldwide Compatible, Power Conversion AC Adapter, Multi Conversion Plug, C/BF/O/A Type, 3 USB-A and 1 USB-C Port, Compatible with

How much heat energy is required to convert 66.3 g of liquid sulfur dioxide, SO2, at 201.2 K to gaseous SO2 - brainly.com

heat of in 1.77 x 10 water = 4.2 J g- K-, specific latent heat = 336 J g-1. The amount of heat energy required to convert of ice -10°C to water

Work done in converting one gram of ice at 10∘ C into steam at 100∘ C is [Given, specific heat of ice c i =0.5 cal g 1∘ C 1, specific heat




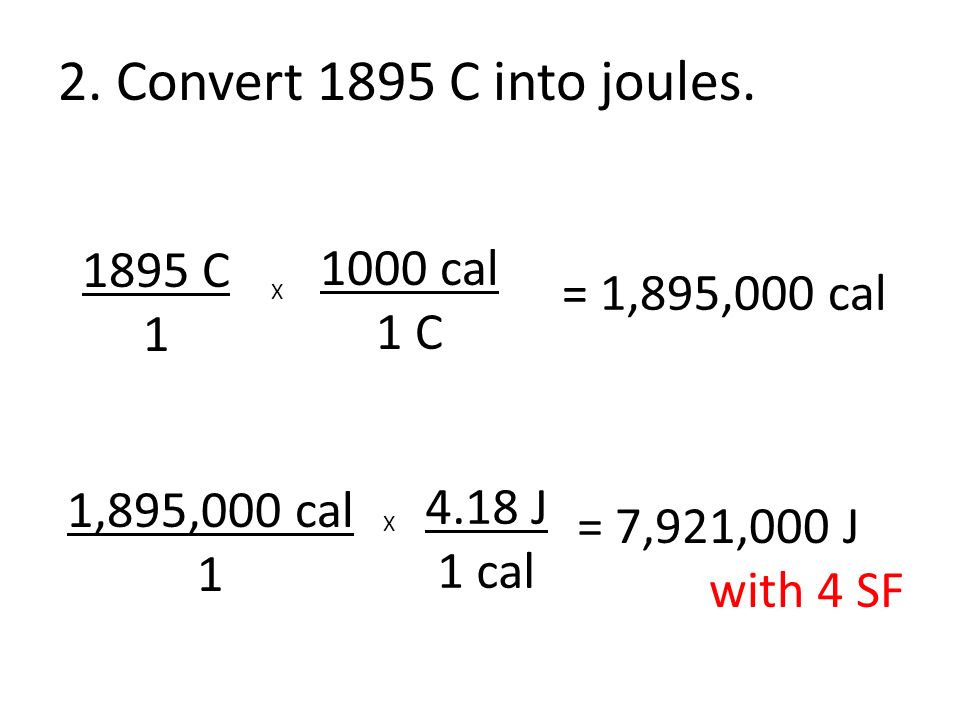
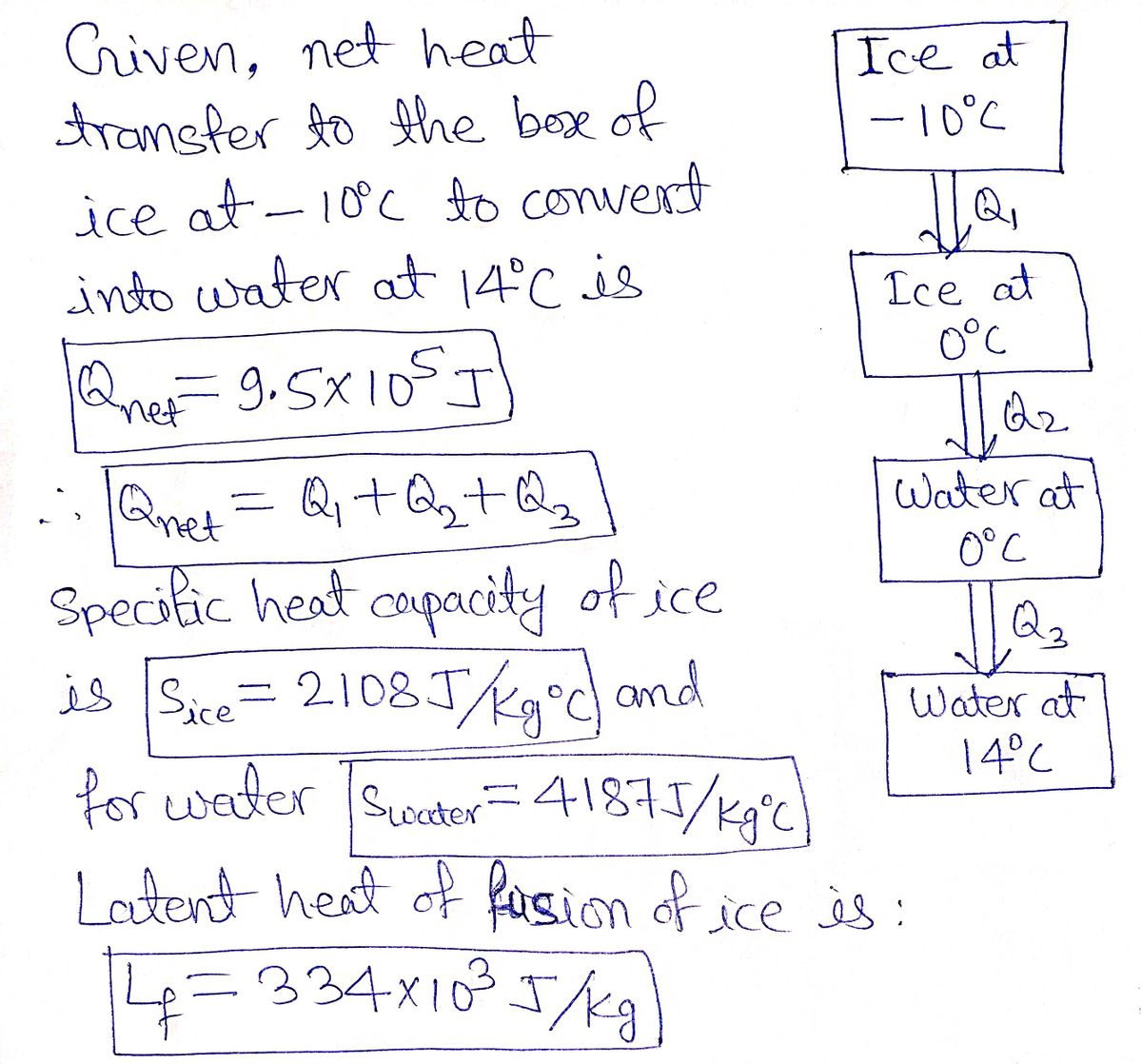





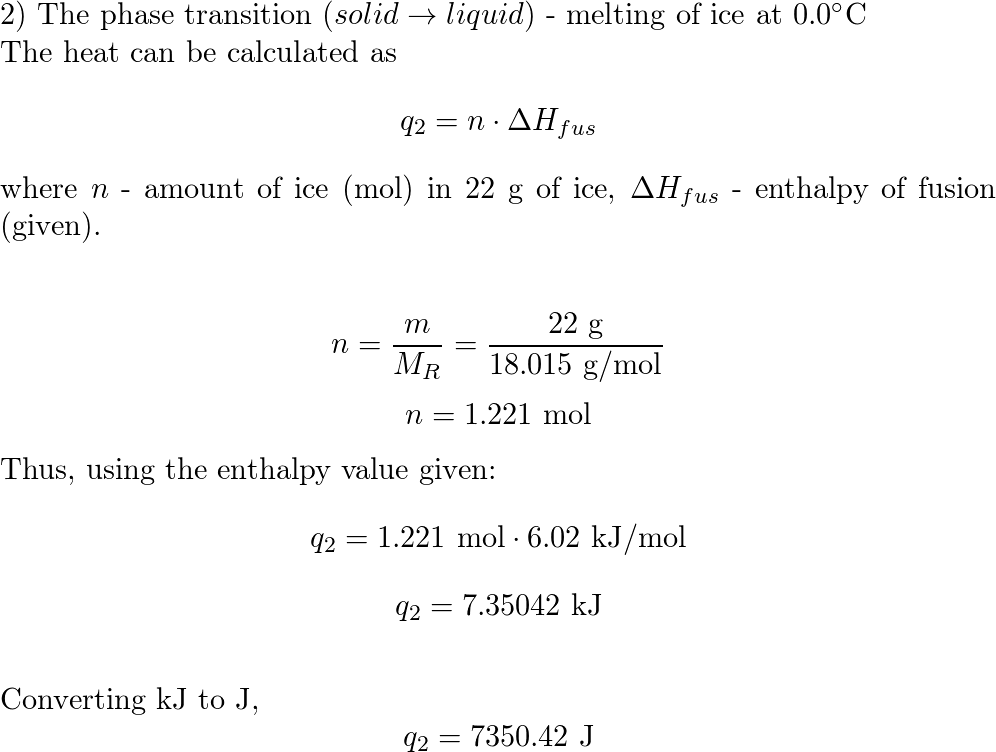
![ANSWERED] What amount of heat (in kJ) is required t... - Physical Chemistry - Kunduz ANSWERED] What amount of heat (in kJ) is required t... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/54105585-1657456603.3299727.jpeg)


